Safety Document Group 3
Occupational Safety Training Materials for Chemical Safety
DOWNLOAD THE OCCUPATIONAL SAFETY DOCUMENT SET (6 GROUPS, OVER 300 PROFESSIONS)
The materials for the occupational safety training course for the chemical industry help workers equip themselves with safety knowledge and prevent hazards when working with chemicals, minimizing chemical accidents, poisoning, burns, or the infiltration of chemicals into internal organs.
PART I: EFFECTS OF CHEMICALS
1. Effects of chemicals on human health
1.1. The toxicity of chemicals in the chemical safety document
The toxicity level of a chemical depends on
- The route of entry of the chemical into the human body
- Chemicals can enter the human body through 3 routes: Respiratory tract; Skin absorption; Digestive tract
- For industrial workers, inhalation is the most common and dangerous route of entry.
- The thickness of the skin, along with sweating and the fatty tissue in the subcutaneous layer, acts as a protective barrier against chemicals entering the body and causing skin damage. Chemicals with solvents that penetrate the skin or are fat-soluble can easily enter the body through the skin. The more fat-soluble a substance is, the higher its toxicity to the nervous system.
- Typically, chemical absorption through the digestive tract is less than the other two routes, and toxicity is reduced when passing through the digestive system due to the action of gastric and pancreatic juices.
- The type of chemical exposed to
- Due to the physicochemical reactions of the toxic substance with the corresponding organ systems, there is a specific distribution for each substance:
- Electrolytic chemicals such as lead and barium concentrate in the bones, while silver and gold are in the skin or deposited in the liver and kidneys as complex compounds.
- Non-electrolytic, fat-soluble organic solvents concentrate in fatty tissues like the nervous system.
- Non-electrolytic substances that are not soluble in fats have a lower ability to penetrate body tissues, depending on molecular size and the concentration of the toxic substance.
- Depending on their physical, chemical, and biological properties, some hazardous chemicals will be eliminated from the body:
- Through the intestines: mainly heavy metals.
- Through bile: Some toxic substances are metabolized and then conjugated with sulfo or glucuronic acid before being excreted through bile.
- A large number of toxic substances in gas or vapor form can be eliminated through breathing.
- Toxic substances can also be eliminated through the skin and breast milk.
- Concentration and duration of exposure
- Short-term exposure to high concentrations of chemicals can cause acute effects (acute poisoning).
- Meanwhile, long-term exposure to low concentrations can lead to two trends: either the body can tolerate it, or the chemical accumulates in larger quantities, leaving chronic effects.
- Combined effects of chemicals

- Sensitivity of the exposed person
- There are significant differences in how individuals react to chemical exposure. With the same amount of exposure over the same period, some people are severely affected, some are mildly affected, and some may show no outward signs.
- Factors that increase the risk of workers being poisoned
- Microclimate:
- High temperature: increases the evaporation rate of toxic substances, increases circulation and respiration, thereby increasing the absorption of toxins.
- Increased air humidity: increases the decomposition of some chemicals with water, increases the accumulation of gases in the mucous membranes, and reduces toxin elimination through sweat, thus also increasing the risk of poisoning.
- Excessive physical labor increases circulation, respiration, and the level of poisoning.
- Inadequate or unbalanced nutrition reduces the body’s resistance…
- Microclimate:
1.2. Harmful effects on humans in the chemical safety document
- Irritation causing discomfort.
- Skin irritation: When a chemical comes into contact with the skin, it can alter the protective layers, causing the skin to become dry, rough, and sore. This condition is called dermatitis.
- Eye irritation: Chemicals in the eyes can cause effects ranging from mild, temporary discomfort to long-term injury. Common eye irritants include acids, alkalis, and solvents.
- Respiratory tract irritation:
- Soluble substances such as ammonia, formaldehyde, sulfur dioxide, acids, and alkalis in the form of mist, gas, or vapor can cause a burning sensation upon contact with the upper respiratory tract (nose and throat); they are absorbed due to the moisture in the nasal passages.
- Less water-soluble chemicals can penetrate the gas exchange region. These substances are less common in the workplace, but the damage they cause to workers is very serious. The reaction of chemicals with lung tissue causes pulmonary edema (fluid in the lungs), which can appear immediately or after several hours. Symptoms begin with severe discomfort in the lungs, followed by coughing, difficulty breathing, cyanosis, and excessive sputum. These chemicals often include: Nitrogen dioxide, ozone, phosgene…
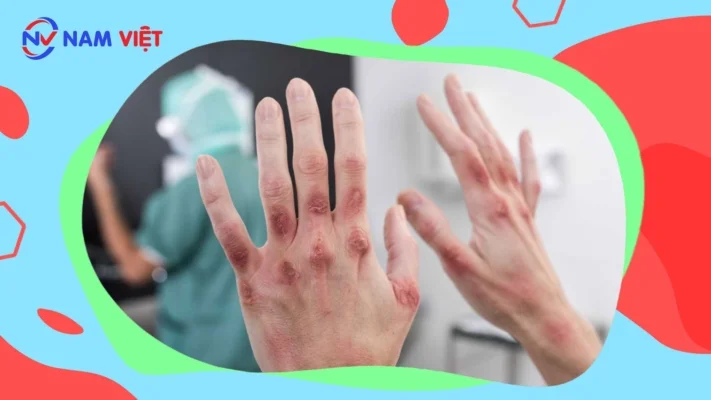
- Causing allergies.
- Skin allergy: Allergic skin has a condition similar to dermatitis.
- Respiratory allergy: is the cause of occupational asthma, with symptoms such as severe coughing at night, difficulty breathing, wheezing, and shortness of breath.
- Causing asphyxiation.
- Simple asphyxiation
- Simple asphyxiants are usually in gaseous form, such as: CO₂, CH₄ (methane), N₂, C₂H₆, H₂…;
- In confined spaces with poor ventilation, these substances displace oxygen, creating an oxygen-deficient environment.
- Simple asphyxiation
| OXYGEN CONCENTRATION | SYMPTOMS |
| < 18 % | Causes asphyxiation |
| 12 – 16% | Rapid breathing |
| 10-14% | Unusual fatigue |
| 6-10% | Nausea and loss of self-control |
| < 6% | Convulsions and respiratory failure, may lead to death |
-
- Chemical asphyxiation
- Chemical asphyxiants prevent the blood from transporting oxygen to the body’s tissues. A typical substance is carbon monoxide.
- Other substances like hydrogen cyanide or hydrogen sulfide… interfere with the cells’ ability to receive oxygen, even when the blood is rich in oxygen.
- Anesthesia and narcosis.
- Chemicals such as: ethanol, propanol (fatty alcohols), acetone and methyl ethyl ketone (fatty ketones), acetylene, hydrocarbons, ethyl and isopropyl ether…
- Exposure to high concentrations can depress the central nervous system, causing fainting and even death. With regular exposure to low concentrations of these chemicals, some people become addicted to them. These substances have effects similar to alcohol intoxication.
- Impact on organ systems.
- Liver:
- Cleans toxins from the blood by converting them into non-toxic, water-soluble substances before excretion. However, some chemicals can damage the liver, leading to cirrhosis and reduced liver function. Symptoms of hepatitis include jaundice (yellowing of the skin and eyes). Solvents include: alcohol, carbon tetrachloride, trichloroethylene, chloroform.
- Kidneys:
- The kidneys are part of the urinary system, whose function is to excrete waste products generated by the body, maintain water and salt balance, and control and maintain the acid concentration in the blood. Chemicals that hinder the kidneys’ ability to excrete toxins include ethylene glycol, carbon disulfide, and carbon tetrachloride. Other compounds such as cadmium, lead, turpentine, ethanol, toluene, xylene… will gradually damage kidney function.
- Liver:
- Pneumoconiosis.
- Substances that cause pneumoconiosis often include: crystalline silica, asbestos…
- Chemical asphyxiation
2. Harm to the environment in the chemical safety document
In industrial activities and the transport of hazardous substances, based on the consequences of accidents, they are divided into 2 main groups:
- Workplace accidents.
- The consequence of a workplace accident is always harm to one or more workers. Usually, workplace accidents are limited to harming the victim and are often not complex. This type of accident occurs due to violations of safety rules. This type of accident is easily predictable and can be prevented or limited through the implementation of safety measures.

- Industrial accidents:
- The consequences of industrial accidents are much greater than those of occupational accidents. The consequences of industrial accidents affect workers, the plant, the population, and the environment. The consequences are initiated by a system component failure, operator or worker error, or by external impacts (dangerous natural phenomena or destructive human actions).
- This type of accident occurs when hazardous events happen: fire, explosion, release of hazardous substances.

3. Some chemicals harmful to humans and the environment.
- Flammable and explosive substances
- Liquids:
- Hydrocarbon solvents (gasoline, kerosene, benzene – C₆H₆, toluene – C₆H₅OH…): flammable
- Alcohols (methanol – CH₃OH, ethanol – C₂H₅OH): flammable
- Ethers, ketones (diethyl ether, acetone – CH₃COCH₃…): flammable
- Carbon disulfide CS₂: flammable
- Hydrogen peroxide – H₂O₂: easily decomposes and explodes when subjected to impacts such as strong light, shock…
- Flammable and explosive solids:
- Phosphorus, sulfur… can ignite in the air at high temperatures.
- Chlorate salts (potassium chlorate, sodium chlorate): are very strong oxidizing agents. If ground with flammable substances, an explosion will occur, for example, a mixture of charcoal powder or sulfur with potassium chlorate will explode if impacted.
- Ammonium nitrate (NH₄NO₃): easily decomposes at high temperatures due to impurities (organic dust, iron oxide, metal fragments…) and can cause an explosion. In dry form, it can explode when ignited.
- Gaseous substances:
- Gases from the hydrocarbon series
- Liquids:
- Toxic substances
- Asphyxiating gases: notably CO, CO₂…
- Other highly toxic substances: NH₃, Cl₂, HCl, SO₂, NOₓ, …; benzene, HCN, CH₃OH…; cyanide salts, soluble mercury salts, lead and its salts…
- Substances that react strongly with water
- Alkali and alkaline earth metals:
- Na, K, Ca, Ba… react with water to produce an alkaline solution, H₂ and a large amount of heat, which can ignite H₂ and cause an explosion.
- Alkali metal peroxides
- NaO, Na₂O₂, K₂O, K₂O₂, … produce O₂ and very strong heat.
- Metal hydrides:
- NaH, CaH₂ react with water to produce H₂ and heat.
- Metal phosphides
- react with water to produce PH₃ (phosphine), a toxic substance that can spontaneously ignite.
- Calcium carbide, aluminum carbide
- CaC₂ reacts with water to produce acetylene.
- Al₄C₃ reacts with water to produce methane.
- Alkali and alkaline earth metals:
Part II: Some measures to prevent and limit harmful factors in the production and use of hazardous chemicals
1. The principle of substitution in the chemical safety document
The best way to prevent or minimize the harm of chemicals to humans and the environment is to avoid using them if less toxic, less hazardous alternatives are available.
- Examples of substituting hazardous chemicals:
- Using water-soluble products or adhesives instead of solvent-based products or adhesives;
- Examples of process substitution:
- Replacing spray painting with electrostatic or dip painting methods;
- Applying mechanical material feeding methods instead of manual feeding.
- A typical example:
- In the garment industry, organic solvents are used to clean raw materials or finished products. Organic solvents are very hazardous and expensive. Contact with cleaning solvents can cause fatigue, headaches, dizziness, eye pain, difficulty breathing, and affect the lungs. If oil stains are cleaned with a solution containing 5-10% soap, it will be cheaper and less toxic than organic solvents.
2. Establish a safe distance or shielding between workers and chemicals
An ideal production process is one where workers’ exposure to chemicals is minimized by
- Completely enclosing machinery, dust-generating points of conveyor belts, or enclosing the production process of corrosive substances… to limit the spread of harmful and dangerous vapors and gases into the working environment.

- Moving the processes and stages of producing these chemicals to a safe location, far from workers in the factory, or building walls to isolate them from other production processes with normal working conditions. For example, isolating the spray painting process from other production processes in the factory with walls or barriers… or placing the painting area downwind.

- In addition, to ensure fire and explosion safety, it is necessary to isolate flammable and explosive chemicals from heat sources, such as placing explosives far from grinding machines, saws…
3. Reduce concentration (ventilation) in the chemical safety document
For volatile chemicals, ventilation is considered the best form of control after substitution or enclosure. With appropriate ventilation equipment, one can prevent dust, vapors, and toxic gases from escaping the production process into the breathing zone of workers and transport them through ducts to a treatment unit (cyclone, settling device, electrostatic precipitator…) for detoxification before being released into the environment.
The ventilation system can be arranged as: a local ventilation system right at the source of vapor/gas generation; or a general ventilation system for the entire factory; or a combination of both systems.
- Local supply system
- Also known as an air shower, it is usually arranged to blow clean, cool air into the fixed workstations of workers where harmful gases and vapors and a lot of heat are often emitted.

- For local exhaust systems
- The system’s exhaust hood must be placed as close as possible to the source of dust, vapor, and toxic gas generation to prevent its harmful effects on nearby workers. There are local ventilation systems that are very effective in controlling toxic substances such as lead, asbestos, and organic solvents.
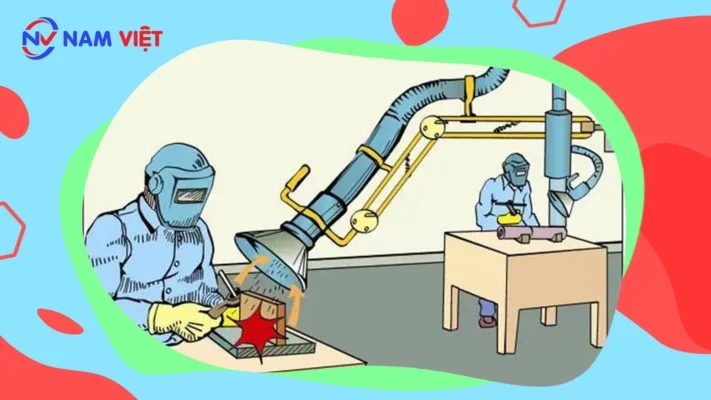
- General ventilation system
- This is a system for diluting the concentration of chemicals. It works on the principle of diluting the air containing dust or chemical vapors by bringing in clean air from the outside and removing dirty air from the production area.
- This can be done by:
- Opening windows and doors to create natural air circulation. The arrangement of these airflows must be done right from the building design stage.
- The method of forced ventilation by machine has the advantage over natural ventilation that the concentration of hazardous chemicals in the intake and exhaust air can be controlled.
- The ventilation method only serves to dilute toxins rather than remove them from the work environment. Therefore, this system is only recommended for substances with low toxicity, non-corrosive, and in small quantities.

4. Equipping Personal Protective Equipment in the chemical safety document
The technical measures mentioned above can control most of the risks from chemical use, but a small amount can still escape into the environment. Thus, the concentration of chemicals in the environment may still not meet the permissible standards, at which point workers must be equipped with personal protective equipment.
This equipment only cleans the contaminated air before it enters the body; it does not reduce or eliminate toxins in the surrounding environment. Therefore, using damaged or incorrect types of protective equipment means direct exposure to hazardous chemicals. Therefore, personal protective equipment should not be considered the first measure to control risks but only as an additional support for technical control measures.
For fire and explosion risks, there is really no equipment that can ensure the safety of workers.
- Respiratory protection
- Filtering-type masks:
- Disposable activated carbon masks: used to filter chemical vapors at low concentrations or to deodorize.
- Filtering-type masks:

-
-
- Masks with replaceable filters:
- Filters are used with half masks, full-face masks, or hoods covering the entire head and ears.
- The filter consists of:
- Filter cartridge: a small metal or plastic box (D = 7-10 cm; H = 2.5 – 4 cm; V = 50 – 200 cm²) containing filtering material.
-

-
-
-
- Small filter canister: volume 250 – 500 cm³, mounted directly onto the mask with a screw thread.
- Large filter canister: volume up to 1000 – 2000cm³, the canister is placed separately in a bag worn on the hip or in front, and the canister is connected to the mask by a flexible, elastic hose.
- Usage requirements
- Each type of filter can filter one or several specific types of toxic gases/vapors.
- Service life: each filter only maintains its filtering efficiency for a certain period. Only to be used when oxygen concentration is >19.5% and if the toxic gas concentration is <2% (for ammonia <3%) use a large canister, <1% use a small canister, <0.1% use a cartridge.
-
- Supplied-air respirators
- The principle of this mask is not to use the ambient air but to use air from a source far from the contamination.
- Free-flow supplied-air respirator: Air is drawn into the mask by inhalation.
- Compressed air supplied-air respirator: Air is supplied to the mask by an air compressor.
- Usage requirements:
- Used in oxygen-deficient environments, with no restriction on usage time.
- Can protect against all airborne toxic substances.
- The disadvantage of free-flow respirators is breathing resistance, so the minimum inner diameter is required to be 2.5 cm and the length can be over 15 m.
- The general disadvantage is the limited range of movement.
- The principle of this mask is not to use the ambient air but to use air from a source far from the contamination.
- Filter mask combined with compressed air supply
- Structure
- Combines the advantages of a filtering-type mask (mobile, simple to use) and a supplied-air respirator (sealed, low breathing resistance).
- Operating principle:
- Air containing toxins is passed through a filter to be cleaned and then supplied back to the mask by a mini compressor powered by a battery. The filter and compressor are worn on a belt.
- Structure
- Self-contained breathing apparatus (SCBA)
- Operating principle:
- An SCBA does not use ambient air but uses its own source of air or O₂ mixture provided by the apparatus. The source can be a compressed cylinder (O₂ or air) or O₂ generated from an oxygen-producing unit.
- Classification:
- Closed-circuit apparatus: Exhaled gas is retained and mixed with O₂ to form breathable air.
- Open-circuit apparatus: Exhaled gas is released into the environment.
- Usage requirements:
- Can protect against all toxic gases.
- No restriction on the range of movement but is heavy.
- Limited usage time.
- Note: The user must be trained, and precautionary measures must be taken.
- Operating principle:
-
- Eye protection
- Goggles for protection against liquid splashes
- Lenses: Only need a type without light-filtering properties.
- Frame: Must choose a type where the entire frame contour contacts the face (sealed goggle type). Additionally, to prevent liquid from entering the eyes, the frame must have indirect ventilation (Indirect vents on the frame provide ventilation and prevent fogging); or a fully sealed goggle type.
- Goggles for protection against liquid splashes

-
- Goggles for protection against irritating vapors
- Must choose a fully sealed type of goggle (does not allow air to enter inside).
- Goggles for protection against irritating vapors

Part III: Fire and Explosion Prevention
1. Combustion processes in the chemical safety document
- Definition
- Combustion is a rapid chemical reaction that produces heat and light. For combustion to occur and be sustained, three elements are needed: fuel (combustible material), an oxidizing agent, and an ignition source.
- Some characteristics of the combustion process
- The combustion of a liquid begins when the liquid vaporizes due to heating.
- Many solids must turn into a liquid and then a vapor to burn. Some turn directly into vapor before burning.
- Usually, the oxidizing agent is oxygen, as combustion reactions often take place in the air.
- Some combustion can occur without the necessary presence of fuel, oxygen, and an ignition source.
- Some substances spontaneously ignite at room temperature: white phosphorus, phosphine, aluminum dust… or organic materials like oily rags spontaneously igniting when exposed to the sun, or fermentation when damp grass is baled and stored in a warehouse.
- Some substances like sodium, potassium metal, calcium carbide… can ignite upon contact with water.
- Some strong oxidizing agents like potassium permanganate, potassium dichromate… can cause a fire if they come into contact with organic substances like oil, grease, sugar… or hydrogen can burn in chlorine without oxygen, or pure oxygen can cause a fire on contact with oil, grease…
- Flammability limits
- The air and fuel must be in a suitable ratio before igniting and causing a fire. If outside this ratio range, combustion will not occur. The lower flammability limit is the lowest concentration of a combustible substance in the air at 25°C that can cause combustion. The upper flammability limit is the highest concentration of a combustible substance in the air at 25°C that can cause combustion.
- Flash point
- This is the lowest temperature at which a flammable liquid gives off enough vapor to form a combustible mixture, which will ignite if an ignition source is present, but the combustion will not be sustained if the source is removed.
- The fire point is the lowest temperature at which a liquid gives off enough vapor to form a combustible mixture that, if ignited, will continue to burn even after the ignition source is removed. It is usually a few degrees higher than the flash point.
2. Explosion processes in the chemical safety document
An explosion is a process that releases energy very rapidly, leading to a very large and sudden expansion of air.
For a chemical explosion to occur, there must first be a combustible substance, a concentration within the flammability limits, and a sufficiently large amount of the substance. Thus, when a combustible substance is released into the air, it will not ignite immediately but needs some time to mix with the air and form a vapor cloud before exploding.
The explosion process occurs in a very short time. The area covered by the vapor cloud will be subjected to a very strong thermal impact, while the thermal radiation in the area outside the cloud decreases sharply and can be ignored. The most dangerous impact of a vapor cloud explosion is the creation of a shock wave that causes damage to people and property.
Special caution must be exercised with compressed gases stored in pressure vessels, as fire and explosion can occur when the container has defects and often lead to serious accidents. Typically, a pressure vessel explosion is followed by a vapor cloud explosion if an ignition source is present.
3. Ignition sources for fire and explosion in the chemical safety document
- Electric current
- Overloaded wires: When current passes through a wire with an insufficient cross-section to carry the load, or at loose connections and contact points, the result is either sparks, a short circuit, or the wire heating up. The temperature of the wire can reach a point sufficient to ignite combustible vapors in the air, ignite flammable materials, or raise the temperature of nearby chemicals to their flash point and cause them to burn.
- Electric arcs, sparks: Often created by short circuits in switches or junction boxes due to broken or uninsulated wires between the positive and negative conductors. The result is heat generation, which can ignite flammable vapors and cause a fire. Molten steel from an electric arc can also ignite flammable materials and heat up flammable chemicals.
- Static electricity
- The charge of static electricity has a high potential difference and can produce very dangerous sparks.
- Static electricity can be generated when two different surfaces come close and then separate. A charge can also build up when flammable liquids are transferred from one container to another without a ground wire.

-
- When two different surfaces come close and are separated, leading to a buildup of charge.
- Heat generated when mixing two chemicals

-
- Mixing two or more chemicals can generate heat.
- Heat generated by friction
- When two surfaces rub against each other, heat can be generated. This is heat generated by friction. The friction of a drive belt against its guard or between two metal surfaces can generate enough heat to ignite combustible vapors. The cause of friction is often a lack of necessary maintenance, leading to the loss of guards or insufficient lubrication on contacting metal surfaces. Sparks can also occur when a stone embedded in a shoe sole rubs against a concrete surface.
- Thermal radiation
- Heat from furnaces, stoves, and other hot surfaces can ignite combustible vapors. The normal production processes of a factory can also generate enough heat to bring nearby stored chemicals to their ignition point and ignite their vapors. Direct sunlight, either by itself or magnified by plastic or glass, can also have this effect.
- Open flames
- Unshielded flames from cigarettes, matches, welding torches, and internal combustion engines are very significant heat sources. When combined with sufficient fuel and oxygen, they can cause fires and explosions.

- Flames from a cutting torch
- can ignite fuel vapors and gases.
- Spontaneous combustion
- sawdust, metal filings, metal shavings, and other types of filings and shavings soaked in vegetable oil or animal fat
- coal, lignite
- cotton fibers
- cloth soaked in linseed and hemp oil
This combustion occurs after several hours or months from the time the materials are left undisturbed. The temperature initially rises slowly, sometimes pausing, but in the final stage, after reaching a certain value, the temperature rises very rapidly and spontaneous combustion occurs.
4. Principles of Fire and Explosion Prevention in the Chemical Safety Document
- Eliminating the formation of a combustible environment
- In technological processes that may release flammable vapors or gases, measures must be taken to eliminate the possibility of creating a combustible environment.
- Use solvents that are less volatile and less flammable.
- Isolate flammable stages in areas far from other equipment and stages. Place them in cool, well-ventilated areas.
- Liquids and gases must be stored in sealed tanks. Storage warehouses must comply with fire safety regulations.
- Prevent the formation of combustible concentrations caused by dust.
- Preventing the formation of ignition sources
5. Principles of Firefighting and Explosion Control
- Firefighting Principles
- Based on the conditions for the combustion process to occur.
- If the thermal equilibrium in the chain reaction zone of the fire is disrupted, those reactions will not occur and the fire will be extinguished.
| Reduce heat generation intensity in the combustion reaction zone | Reduce pressure in the combustion reaction zone |
| Change the concentration of components involved in the combustion reaction | |
| Chemically inhibit the combustion reactions | |
| Enhance heat dissipation from the combustion reaction zone | Increase the emissivity of the combustion reaction zone |
| Increase the thermal conductivity from the combustion reaction zone | |
| Reduce the ambient temperature around the combustion reaction zone | |
| Both of the above solutions simultaneously |
- Fire Extinguishing Agents
- Water
- The fire extinguishing mechanism of water is to cool the combustion reaction zone. When water vaporizes, it carries away a very large amount of heat.
- Some cases where water should not be used:
- Fighting electrical equipment fires because water can conduct electricity.
- Do not use water to extinguish fires with high flame temperatures (1700°C), as water decomposes to form explosive gases (O₂ and H₂).
– Do not use water to extinguish fires of liquids that are immiscible with water, as well as liquids lighter than water.
- Substances that react with water to produce flammable substances, oxygen (alkali metals, alkaline earth metals, metal carbides, coal…).
- Water
- Firefighting Foam
- Chemical firefighting foam: Part A is aluminum sulfate Al₂(SO₄)₃, Part B is NaHCO₃. When they react, they produce CO₂. The firefighting mechanism is to isolate the components involved in the combustion reaction, as well as cooling the fire zone and reducing the concentration of the reactants.
- Air-aspirating foam: Foam is formed by mixing air with a foaming solution. Air can be replaced with other non-combustible gases like CO₂.
- Used to fight gasoline, oil, and flammable liquid fires.
- Dry Powder Extinguisher
- These are non-combustible mineral salts in solid form.
- Firefighting mechanism
- Reduces the concentration of components involved in the combustion reaction.
- Absorbs heat in the combustion reaction zone.
- Inhibits the combustion reaction through a barrier mechanism.
- Chemically inhibits the combustion reactions.
- Isolates the components involved in the combustion reaction.
- Non-combustible Gases
- Mechanism
- Dilutes the combustible concentration.
- Cools the fire zone.
- Used for fighting fires on energized electrical equipment and solids that cannot be extinguished with water.
- Note: Do not use to fight fires of substances that can create new flammable or explosive materials.
- Mechanism
| FIRE CLASS | CHARACTERISTICS OF FIRE MATERIAL | FIRE GROUP | CHARACTERISTICS OF FIRE GROUP |
| A | Solids | 1 A | Fires involving solids with smoldering combustion (wood, paper, dry grass, straw, coal, textile products) |
| 2 A | Fires involving solids without smoldering combustion (plastics) | ||
| B | Liquids | 1 B | Fires involving liquids insoluble in water (gasoline, ether, petroleum fuels); fires of liquefiable solids (paraffin) |
| 2 B | Fires involving liquids soluble in water (alcohol, methanol, glycerin) | ||
| C | Gases | ||
| D | Metals | 1 D | Fires involving light metals (aluminum, magnesium, and their alloys) |
| 2 D | Fires involving alkali metals and other similar metals (sodium, potassium) | ||
| 3 D | Fires involving metal-containing compounds (organometallic compounds, metal hydrides) |
| EXTINGUISHING AGENT | COMBUSTIBLE MATERIAL | ||||||||
| A | B | C | D | ||||||
| A1 | A2 | B1 | B2 | D1 | D2 | D3 | |||
| Water | Highly suitable | Not suitable | Not suitable | Not suitable | |||||
| Foam | high expansion ratio | Highly suitable | Suitable | Not suitable | Not suitable | Not suitable | |||
| low and medium expansion ratio | Suitable | Not suitable | Highly suitable | Suitable | Not suitable | Not suitable | |||
| Gas | CO2 | Not suitable | Suitable | Suitable | Not suitable | ||||
| N2, Ar… (inert) | Suitable | Suitable | Suitable | Not suitable | |||||
| Powder | BC | Not suitable | Highly suitable | Highly suitable | Not suitable | ||||
| ABC | Suitable | Not suitable | |||||||
| ABCD | Highly suitable | Not suitable | |||||||
6. How to Use Some Common Fire Extinguishing Agents
- CO₂ Fire Extinguisher
- Structure

-
- Uses.
- A CO₂ fire extinguisher is a device containing CO₂ gas compressed into a high-pressure cylinder (60 bar), used for extinguishing fires, with high reliability, simple and convenient operation, and high efficiency.
- CO₂ extinguishers are very effective for fires in enclosed spaces, rooms, basements, and on electrical equipment. After extinguishing the fire, it leaves no residue and does not damage the combustible material.
- How to use and principle of firefighting.
- In case of a fire, carry the CO₂ extinguisher to the fire, hold the nozzle with one hand pointing at the base of the fire from a minimum distance of 0.5m, and open the cylinder valve or squeeze the trigger with the other hand (depending on the type of extinguisher).
- CO₂ gas at a temperature of –79°C in the form of cold snow, when discharged through the nozzle, has the effect of lowering the temperature of the fire (extinguishing by cooling). Then, the CO₂ gas covers the entire surface of the fire, reducing the concentration of oxygen diffusing into the fire zone. When the oxygen content is less than 14%, the fire will be extinguished (extinguishing by dilution).
- Points to note during use and storage of CO₂ extinguishers
- Do not spray CO₂ gas on people as it will cause cold burns. When spraying, the hand holding the nozzle must be on the designated handle (because holding other parts will cause cold burns).
- CO₂ fire extinguishers must be placed in cool, easily accessible, and convenient places for use.
- Check the amount of gas in the cylinder once every three months by weighing it.
- Uses.
- MFZ System Dry Powder Fire Extinguisher.
- Structure
-
- Uses
- The MFZ system dry powder fire extinguisher is a device containing N₂ gas as a propellant to spray dry chemical powder to extinguish fires. The MFZ system dry powder extinguisher is used to fight fires involving gasoline, oil, flammable gases, electrical equipment… It is highly safe to use, simple to operate, easy to inspect, and has high firefighting efficiency.
- How to use
- For portable type:
- Move the extinguisher close to the fire location.
- Shake it a few times if it is a powder type with a combined gas propellant (MFZ).
- Pull the safety pin.
- Choose the upwind direction and aim the nozzle at the base of the fire.
- Squeeze the valve to release the firefighting powder.
- When the gas pressure weakens, move closer and sweep the nozzle back and forth to completely extinguish the fire.
- For wheeled type
- Push the cart to the fire location, pull out the hose reel, and aim the powder nozzle at the base of the fire.
- Pull the safety pin, pull the main valve on top of the cylinder perpendicular to the ground.
- Hold the nozzle firmly, choose the downwind direction, and squeeze the trigger; the powder will be discharged.
- Points to note during use and storage.
- When spraying, stand downwind.
- Place the extinguisher in cool, easily accessible, and convenient places for use.
- Check the extinguisher once every three months. If the pressure gauge needle points to the red zone, the extinguisher must be recharged.
- For portable type:
- Uses
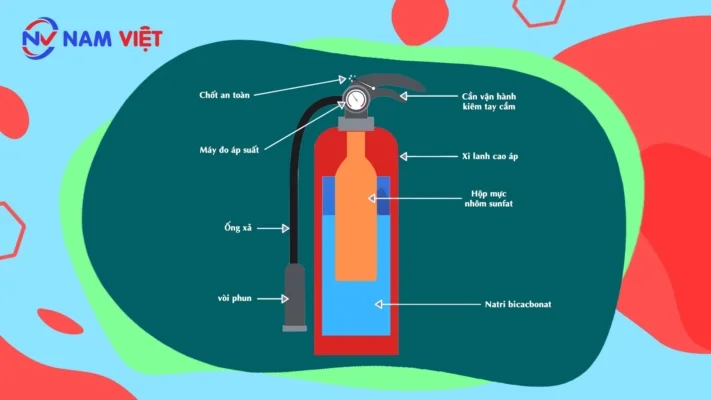
- Chemical Foam Extinguisher
- Structure

-
-
- The extinguisher body is made of steel (20kg/cm²) containing a Na₂CO₃ solution. Inside the body: one container holds H₂SO₄ at a concentration of 65.5%, and another container holds aluminum sulfate at a concentration of 35%.
- How to use
- To fight a fire, bring the extinguisher near the fire, turn it upside down so the pin is pointing down, and gently tap the pin on the floor. The two chemical solutions will mix, react to produce foam, and you can then direct the nozzle at the fire.
-
Part IV: First Aid
1. Chemical Burns in the Chemical Safety Document
Some chemicals can cause injury to the skin and even internal organs. In severe, dangerous cases, it can lead to death. The signs of chemical burns are not like thermal burns, as they develop more slowly. The first aid principles for both types of burns are the same.
Signs to recognize:
- Severe pain.
- After some time, the skin becomes discolored, blistered, and peels.
Treatment:
- Identify and remove the chemical immediately before it causes more damage. Do not hesitate; provide immediate treatment.
- Continuously flush the affected area with water:
- 5 minutes for moderate irritants.
- 20 minutes for severe irritants.
- 20 minutes for non-deeply corrosive substances.
- 60 minutes for deeply penetrating corrosive substances.
- Absolutely do not use any neutralizing agent to remove the chemical (e.g., acid vs. alkali), as it will only worsen the injury.
- Remove any tight clothing from the victim.
- Take the victim for emergency medical care immediately. Provide detailed information about the incident and the victim’s condition to medical personnel. Inform the doctor of the chemical that caused the burn (if known).
2. Chemicals in the Eye
Chemicals getting into the eye can have serious consequences if not treated promptly. It can damage the cornea and leave scars, causing vision loss.
Symptoms:
- Severe pain in the eye.
- Inability to open the injured eye.
- Swelling and redness inside and around the eye.
- Tears are produced.
First Aid:
- Rinse the injured eye under clean running water for about 10 minutes. Be careful not to let the rinsing water get into the uninjured eye.
- If it hurts to close the eye, gently pull the eyelid open. Be careful not to let the rinsing water flow into the other eye.
- Cover the eye with a sterile bandage or clean gauze.
- Take the patient to the hospital immediately.
3. In Case of Poisoning
- Accidentally drinking acid
- Do not perform gastric lavage, do not induce vomiting, do not neutralize with carbonate or bicarbonate salts as this will produce CO₂, causing bloating and risking perforation.
- Give plenty of dilute alkaline solutions to drink, such as soap water, magnesium water (20g/l), or limewater mixed with sugar.
- Poisoning from drinking alkali (e.g., ammonia solution, caustic soda)
- Provide first aid by giving the victim dilute vinegar (2% acetic acid) or lemon juice to drink. Do not induce vomiting or perform gastric lavage.
- Poisoning from inhaling toxic gases like chlorine, bromine (Cl₂, Br₂)
- Move the victim to a well-ventilated area, loosen their belt, and have them breathe air with a small amount of ammonia, or use a mixture of 90°C alcohol with ammonia.
- Poisoning from inhaling hydrogen sulfide, carbon monoxide… (H₂S, CO)
- Move the victim to lie down in a well-ventilated area, have them breathe pure oxygen, and perform artificial respiration if necessary.
- Poisoning from inhaling too much ammonia
- Have the victim inhale hot steam, then give them lemon juice or dilute vinegar to drink.
- The Bhopal disaster of 1984

28 years have passed since India’s worst industrial disaster claimed 15,000 lives and injured 558,125 people. The leak of methyl isocyanate (MIC) gas and other chemicals from the pesticide plant in Bhopal caused an extremely severe disaster for people and the environment. On his way to the scene, renowned Indian photographer Pablo Bartholomew captured the image of a man burying a young victim – an image that tells the entire tragic story of the people of Bhopal.


